How do you calculate the atomic mass of carbon?
- What Is Relative Atomic Mass
- Relative Atomic Mass Of Carbon 12
- Relative Atomic Mass Of Carbon Tetrachloride
- Relative Formula Mass Of Carbon Dioxide
The carbon-based standard represented a nice compromise. By chance, defining the atomic mass as 1/16th of the mass of a mole of oxygen comprising a natural mix of 16 O, 17 O, and 18 O is very close to a standard defining the atomic mass as 1/12 the mass of a mole of 12 C 3. Do you want to know how to calculate Relative Atomic Mass? In this education video by The Fuse School, you are going to learn about:- How to calculate Relati.
1 Answer
The term 'atomic mass' refers to the mass of a single atom. The mass of a single atom of carbon-12 is defined as exactly 12 u.
The term atomic mass is also often used (though technically, incorrectly) to refer to the average atomic mass of all of the isotopes of an element.
This second definition is actually the relative atomic mass of an element — a single average value of the element's mass based on the masses of its isotopes.
Carbon has 15 known isotopes, of which only two (
Carbon consists of 98.93%
METHOD 1
To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms.
Assume that you have, say, 10 000 atoms of carbon. Then you have 9893 atoms of
Media join for mac.
:star: Use repo badges (build passing, coverage, etc) in your readme/markdown file to signal code quality in a project. dwyl/repo-badges. Signal markdown.
METHOD 2
Another way of determining the average mass is to multiply the atomic mass of each isotope by its percentage and then add the numbers.
The two methods are mathematically equivalent. Thus,
What Is Relative Atomic Mass
Method 2 is probably mathematically simpler, but Method 1 makes it clear that you are determining an average mass.
Choose the method that you prefer.
Related questions
Relative Mass
The relative mass of an object is the comparison of the mass of the object to the mass of a standard object.
Keyboard maestro. Keyboard Maestro can help you fill in web forms, visit commonly read pages, download reports or bank statements, format web pages, and generally make browsing the web more efficient. Keyboard Maestro is (simplest definition) an application to launch macros on your Mac. These macros can be used to automate just about any repetitive task, like navigating running applications, opening documents, managing text, and controlling web applications.
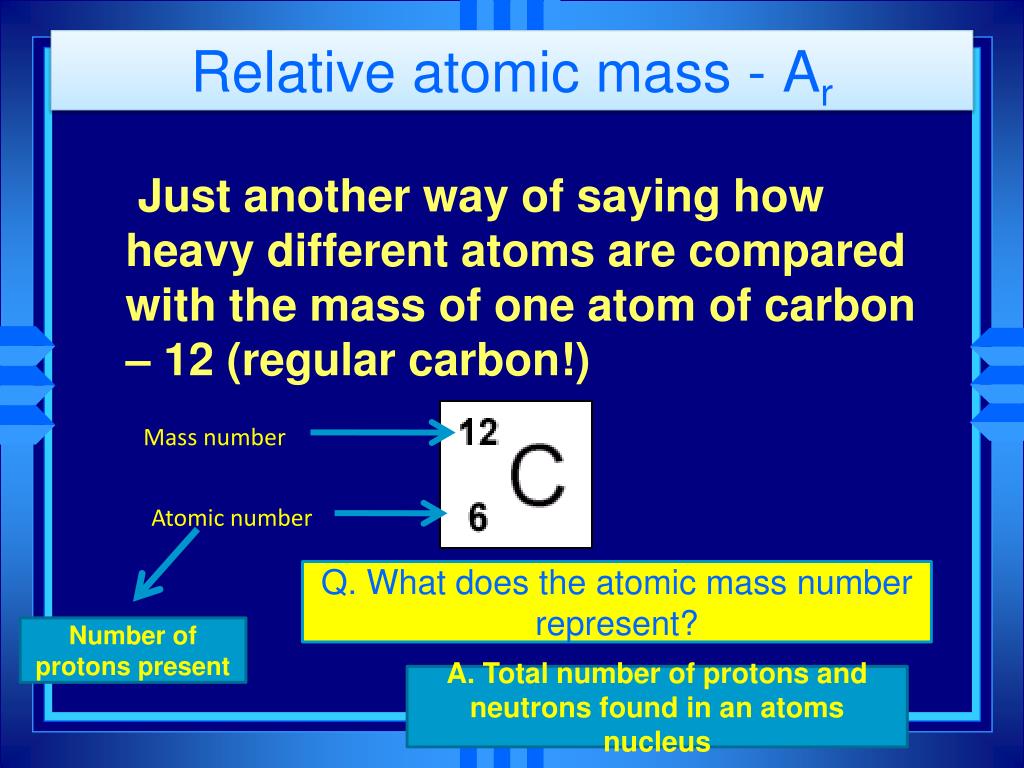
Relative Atomic Mass
Relative Atomic Mass
Relative Atomic Mass Of Carbon 12
The relative atomic mass (Ar) of an element is the average mass of one atom of the element when compared with 1/12 of the mass of an atom of carbon-12, which taken as 12 units.
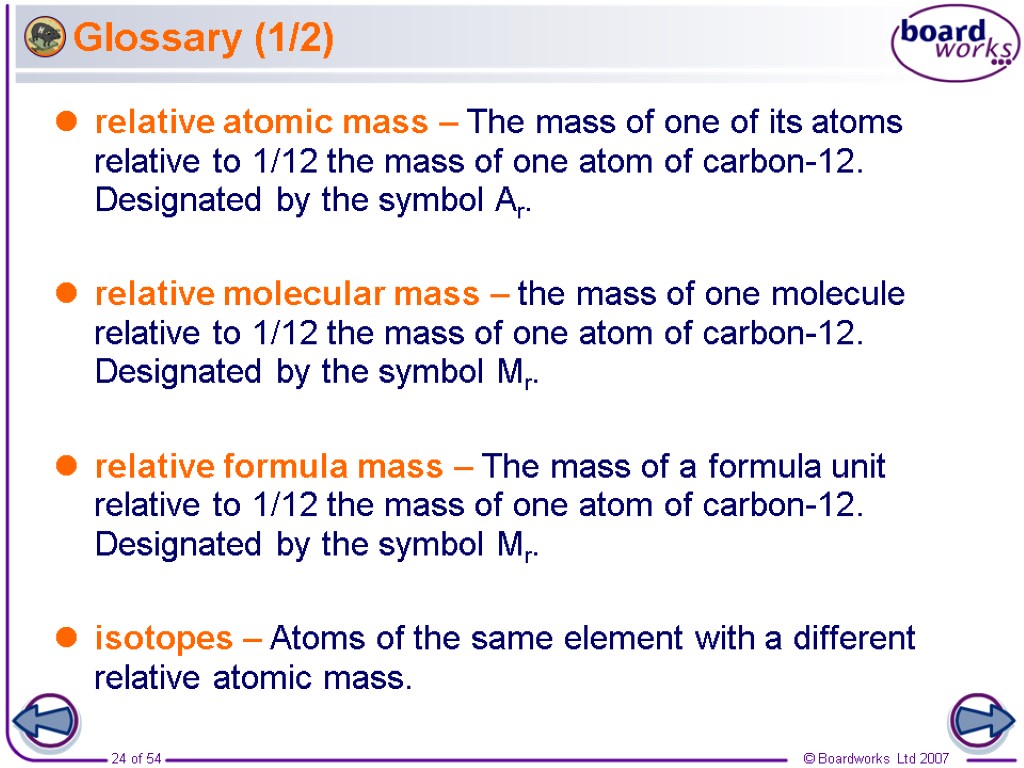
- The mass of an atom when compared to another is known as the relative atomic mass (Ar).
- The relative atomic mass (Ar) of an element is the average mass of one atom of the element when compared with 1/12 of the mass of an atom of carbon-12, which taken as 12 units.
- 1/12 of the mass of an atom of carbon-12 is named as 1 atomic mass unit (amu).
- The mass of one carbon atom is 12 amu.
- 1/12 of the mass of an atom of carbon-12 is named as 1 atomic mass unit (amu).
- The mass of one carbon atom is 12 amu.
Example 1
Example 2:
The mass of element A is twice of the mass of carbon, therefore its relative atomic mass is __________. (Relative atomic mass of carbon = 12)
Answer:
Example 3:
An atom of element X is 13 times heavier than one atom of helium. Calculate the relative atomic mass of X.( Ar: He = 4 )
Answer:
Example 4:
How many times that the mass of 2 bromine atoms are greater than 4 neon atoms? (Ar: Ne = 20; Br = 80 )
Answer:
2(80) / 4(20) =2
Relative Atomic Mass Of Carbon Tetrachloride
Example 5
4 atoms of element L have the same mass as 1 tellurium atom. Find the relative atomic mass of L. (Ar: Te = 128 )
Relative Formula Mass Of Carbon Dioxide
4L = 1(128)
L = 128/4 =32
